
Chemistry, 31.03.2020 00:59 charnaetoney13
Na2CO3(aq)+2HCl(aq)→2NaCl(aq)+H2O(l )+CO2(g) A student combined two colorless aqueous solutions. One of the solutions contained Na2CO3 as the solute and the other contained HCl . The chemical reaction that took place is represented by the equation above. What experimental result would be evidence that a chemical reaction took place when the solutions were combined?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Na2CO3(aq)+2HCl(aq)→2NaCl(aq)+H2O(l )+CO2(g) A student combined two colorless aqueous solutions. One...
Questions


Mathematics, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01




English, 02.10.2020 19:01

Biology, 02.10.2020 19:01



Social Studies, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01



Mathematics, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01


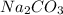 and HCl chemically combine together leading to the formation of NaCl, water and carbon dioxide gas. As the gas is forming and its formation will also form bubbles into the solution.
and HCl chemically combine together leading to the formation of NaCl, water and carbon dioxide gas. As the gas is forming and its formation will also form bubbles into the solution.

