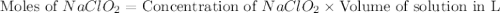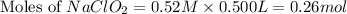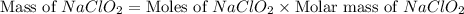Chemistry, 31.03.2020 01:01 nestergurl101
A chemistry graduate student is given 500.mL of a 0.40M chlorous acid HClO2 solution. Chlorous acid is a weak acid with =Ka×1.110−2. What mass of NaClO2 should the student dissolve in the HClO2 solution to turn it into a buffer with pH =2.11?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
A chemistry graduate student is given 500.mL of a 0.40M chlorous acid HClO2 solution. Chlorous acid...
Questions

Biology, 23.10.2020 18:30



Mathematics, 23.10.2020 18:30

Spanish, 23.10.2020 18:30

Mathematics, 23.10.2020 18:30

Mathematics, 23.10.2020 18:30



English, 23.10.2020 18:40


Geography, 23.10.2020 18:40






Mathematics, 23.10.2020 18:40

Mathematics, 23.10.2020 18:40

Biology, 23.10.2020 18:40

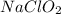 should be, 23.5 grams.
should be, 23.5 grams.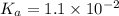
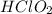 = 0.40 M
= 0.40 M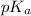 .
.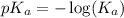
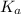 in this expression, we get:
in this expression, we get: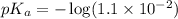

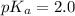
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0572/2296/e961a.png)
![pH=pK_a+\log \frac{[NaClO_2]}{[HClO_2]}](/tpl/images/0572/2296/a8df0.png)
![2.11=2.0+\log (\frac{[NaClO_2]}{0.40})](/tpl/images/0572/2296/3a0b0.png)
![[NaClO_2]=0.52M](/tpl/images/0572/2296/2adfa.png)