
Chemistry, 31.03.2020 00:57 jettskii214
9. Calculate the pH for each of the following cases in the titration of 35.0 mL of 0.200-M methylamine, CH3NH2(aq), with 0.200 M HCl(aq): (a) before addition of any HCl(aq) (b) after addition of 17.5 mL of HCl(aq) (c) after addition of 34.9 mL of HCl(aq) (d) after addition of 35.0 mL of HCl(aq) (e) after addition of 35.1 mL of HCl(aq)

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
9. Calculate the pH for each of the following cases in the titration of 35.0 mL of 0.200-M methylami...
Questions

Mathematics, 05.04.2021 14:00

Social Studies, 05.04.2021 14:00

English, 05.04.2021 14:00


Biology, 05.04.2021 14:00

English, 05.04.2021 14:00


Mathematics, 05.04.2021 14:00


Geography, 05.04.2021 14:00



Mathematics, 05.04.2021 14:00

Computers and Technology, 05.04.2021 14:00

Social Studies, 05.04.2021 14:00

Advanced Placement (AP), 05.04.2021 14:00


Social Studies, 05.04.2021 14:00

History, 05.04.2021 14:00

![K_{b} =\frac{[CH_{3}NH_{3}]+ [OH-] }{[CH_{3}NH_{2} ] }\\4.6x10^{-4} =\frac{x*x}{0.2-x} \\x=0.00936 M](/tpl/images/0572/2081/59e94.png)
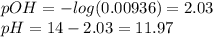
![[acid]=\frac{M_{2}V_{2} }{V_{1}+V_{2} } =\frac{0.2*17.5}{35+17.5} =0.0667M\\[base]=\frac{M_{1}V_{1} }{V_{1}+V_{2} } =\frac{0.2*35}{35+17.5} =0.13M](/tpl/images/0572/2081/75f14.png)
![pOH=pK_{b} +log(\frac{[salt]}{[base]} )=3.34+log(0.0667/0.066)=3.34\\pH=14-3.34=10.66](/tpl/images/0572/2081/0a576.png)
![[acid]=\frac{0.2*34.9}{35+34.9} =0.0998M\\\\ [base]=\frac{0.2*35}{35+34.9} =0.1M\\base-left=0.1-0.0998=0.0002M\\pOH=3.34+log(0.0998/0.0002)=6.04\\pH=14-6.04=7.96](/tpl/images/0572/2081/d31c6.png)
![[acid]=\frac{0.2*35}{35+35} =0.1M\\[base]=\frac{0.2*35}{35+35} =0.1M\\\\pH=7-\frac{1}{2} (pK_{b}+ log(c))=7-\frac{1}{2}(3.34+log(0.1))=5.83 \\](/tpl/images/0572/2081/b3303.png)
![[acid]=\frac{0.2*35.1}{35+35.1} =0.1M\\[base]=\frac{0.2*35}{35+35.1} =0.0998M\\acid-left=0.1-0.0998=0.0002M\\pH=-log(0.0002)=3.7\\](/tpl/images/0572/2081/1cd67.png)


