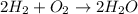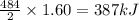Chemistry, 31.03.2020 01:21 LtotheJ0225
Consider the reaction 2 H 2 + O 2 ⟶ 2 H 2 O Δ H rxn = − 484 kJ Which answer best describes the transfer of heat that occurs when 1.60 mol H 2 reacts? 387 kJ absorbed 484 kJ released 387 kJ released 774 kJ absorbed 484 kJ absorbed 774 kJ released

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:50
What does standard deviation reveal about data? a. the average of all the data points b. which of the data points is most reliable c. how spread out the data points are d. the percent error included in the data
Answers: 2

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1
You know the right answer?
Consider the reaction 2 H 2 + O 2 ⟶ 2 H 2 O Δ H rxn = − 484 kJ Which answer best describes the trans...
Questions


Social Studies, 28.08.2020 21:01





Computers and Technology, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01








Mathematics, 28.08.2020 21:01



Mathematics, 28.08.2020 21:01

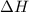 is positive.
is positive.