Chemistry, 31.03.2020 01:26 jladinosolarsee
A 15.5 g piece of chromium, heated to 100.0 degrees Celsius, is dropped into 55.5 g of water at 16.5 degrees Celsius. The final temperature of the metal and the water is 18.9 degrees Celsius. What is the specific heat capacity of chromium

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
A 15.5 g piece of chromium, heated to 100.0 degrees Celsius, is dropped into 55.5 g of water at 16.5...
Questions




Mathematics, 22.04.2020 16:01

Mathematics, 22.04.2020 16:01


Mathematics, 22.04.2020 16:01

English, 22.04.2020 16:01

Computers and Technology, 22.04.2020 16:01

Chemistry, 22.04.2020 16:01



Chemistry, 22.04.2020 16:01



Mathematics, 22.04.2020 16:01

Medicine, 22.04.2020 16:01



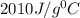
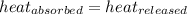
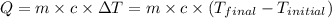
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0572/2921/09236.png) .................(1)
.................(1)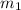 = mass of chromium = 15.5 g
= mass of chromium = 15.5 g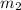 = mass of water = 55.5 g
= mass of water = 55.5 g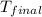 = final temperature =
= final temperature =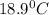
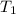 = temperature of chromium =
= temperature of chromium = 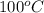
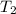 = temperature of water =
= temperature of water = 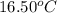
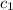 = specific heat of chromium= ?
= specific heat of chromium= ?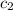 = specific heat of water=
= specific heat of water= 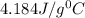
![-15.5\times c_1\times (19.5-18.9)=[55.5\times 4.184\times (19.5-100)]](/tpl/images/0572/2921/a3280.png)