
Chemistry, 31.03.2020 01:29 jaqwannewsome
A metal object with mass of 23.2 g 23.2 g is heated to 97.0 °C 97.0 °C and then transferred to an insulated container containing 90.0 g 90.0 g of water at 20.5 °C. 20.5 °C. The water temperature rises and the temperature of the metal object falls until they both reach the same final temperature of 22.6 °C. 22.6 °C. What is the specific heat of this metal object? Assume that all the heat lost by the metal object is absorbed by the water.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
A metal object with mass of 23.2 g 23.2 g is heated to 97.0 °C 97.0 °C and then transferred to an in...
Questions

Mathematics, 17.08.2020 14:01

Physics, 17.08.2020 14:01

Mathematics, 17.08.2020 14:01


Geography, 17.08.2020 14:01


History, 17.08.2020 14:01


Mathematics, 17.08.2020 14:01


Health, 17.08.2020 14:01

Mathematics, 17.08.2020 14:01



Mathematics, 17.08.2020 14:01


Business, 17.08.2020 14:01

Mathematics, 17.08.2020 14:01

Mathematics, 17.08.2020 14:01

Mathematics, 17.08.2020 14:01

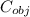 = 0.457
= 0.457 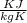
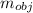 = 23.2 gm
= 23.2 gm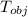 = 97 ° c
= 97 ° c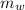 = 90 gm
= 90 gm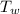 = 20.5 ° c
= 20.5 ° c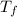 = 22.6 ° c
= 22.6 ° c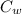 (
( 

