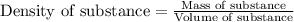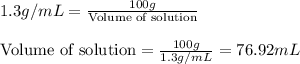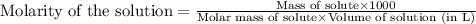Chemistry, 31.03.2020 01:50 jazminjb83
We have an aqueous solution that contains 23% (by mass) of a hypothetical solute Z. The formula weight of the solute Z is 139 g/mol. The density of the solution is observed to be 1.3 g/mL. What is the molarity of Z in this solution

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
We have an aqueous solution that contains 23% (by mass) of a hypothetical solute Z. The formula weig...
Questions


English, 22.07.2019 05:30







Advanced Placement (AP), 22.07.2019 05:30


Social Studies, 22.07.2019 05:30

History, 22.07.2019 05:30

English, 22.07.2019 05:30

Physics, 22.07.2019 05:30



Spanish, 22.07.2019 05:30

Health, 22.07.2019 05:30