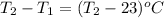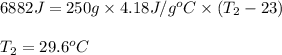The ΔH for the solution process when solid sodium hydroxide dissolves in water is 44.4 kJ/mol. When a 6.21-g sample of NaOH dissolves in 250.0 g of water in a coffee-cup calorimeter, the temperature increases from 23.0 °C to °C. Assume that the solution has the same specific heat as liquid water, i. e., 4.18 J/g-K.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
You know the right answer?
The ΔH for the solution process when solid sodium hydroxide dissolves in water is 44.4 kJ/mol. When...
Questions

Mathematics, 24.11.2020 07:30



Mathematics, 24.11.2020 07:30


Business, 24.11.2020 07:30



Mathematics, 24.11.2020 07:30

Mathematics, 24.11.2020 07:30

Biology, 24.11.2020 07:30





English, 24.11.2020 07:30

English, 24.11.2020 07:30

Mathematics, 24.11.2020 07:30


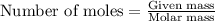
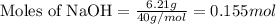
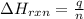
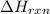 = enthalpy change of the reaction = 44.4 kJ/mol = 44400 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy change of the reaction = 44.4 kJ/mol = 44400 J/mol (Conversion factor: 1 kJ = 1000 J)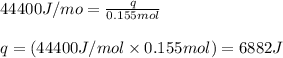
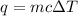
 = change in temperature =
= change in temperature =