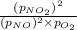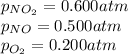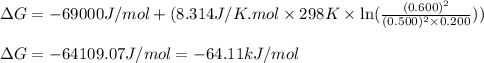For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all species. For the reaction 2 NO ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO 2 ( g ) the standard change in Gibbs free energy is Δ G ° = − 69.0 kJ/mol . What is ΔG for this reaction at 298 K when the partial pressures are P NO = 0.500 atm , P O 2 = 0.200 atm , and P NO 2 = 0.600 atm ?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3


Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all specie...
Questions

Computers and Technology, 24.04.2020 22:54


Mathematics, 24.04.2020 22:54


Mathematics, 24.04.2020 22:54

Mathematics, 24.04.2020 22:54

Mathematics, 24.04.2020 22:54



Biology, 24.04.2020 22:54

Mathematics, 24.04.2020 22:55

Chemistry, 24.04.2020 22:55

Mathematics, 24.04.2020 22:55

Biology, 24.04.2020 22:55



Mathematics, 24.04.2020 22:55



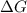 for the given reaction at 298 K is -64.11 kJ/mol
for the given reaction at 298 K is -64.11 kJ/mol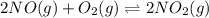
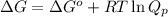
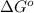 = Standard Gibbs free energy = -69.0 kJ/mol = -69000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Standard Gibbs free energy = -69.0 kJ/mol = -69000 J/mol (Conversion factor: 1 kJ = 1000 J)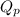 = Ratio of partial pressure of products and reactants =
= Ratio of partial pressure of products and reactants =