
The rate constant for a first-order reaction is 0.54 s-1. What is the half-life of this reaction if the initial concentration is 0.54 M? The rate constant for a first-order reaction is 0.54 s-1. What is the half-life of this reaction if the initial concentration is 0.54 M? 4.7 s 0.49 s 1.0 s 1.3 s 1.8 s

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
The rate constant for a first-order reaction is 0.54 s-1. What is the half-life of this reaction if...
Questions

Mathematics, 21.08.2019 15:50

Social Studies, 21.08.2019 15:50

English, 21.08.2019 15:50

Mathematics, 21.08.2019 15:50


History, 21.08.2019 16:00

History, 21.08.2019 16:00






Health, 21.08.2019 16:00






History, 21.08.2019 16:00

Mathematics, 21.08.2019 16:00

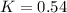
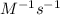
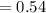 M
M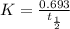
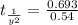
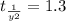 sec
sec

