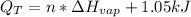Ethanol boils at 78.4 °C with \DeltaΔHvap = 38.6 kJ/mol. A 0.200-mol sample of ethanol is heated from some colder temperature up to 78.4 °C, which requires 1.05 kJ of heat, and then vaporized. What will be the total amount of heat required (for both the heating and the vaporizing)?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Ethanol boils at 78.4 °C with \DeltaΔHvap = 38.6 kJ/mol. A 0.200-mol sample of ethanol is heated fro...
Questions

Mathematics, 04.07.2019 02:30

Biology, 04.07.2019 02:30

Mathematics, 04.07.2019 02:30

History, 04.07.2019 02:30

Mathematics, 04.07.2019 02:30

Mathematics, 04.07.2019 02:30



Mathematics, 04.07.2019 02:30

Chemistry, 04.07.2019 02:30

Mathematics, 04.07.2019 02:30


Mathematics, 04.07.2019 02:30

Mathematics, 04.07.2019 02:30

Biology, 04.07.2019 02:30

Physics, 04.07.2019 02:30




Mathematics, 04.07.2019 02:30