Chemistry, 01.10.2019 19:50 justinhk10
Exactly 313.5 j will raise the temperature of 10.0 g of a metal from 25.0 c to 60.0 c. what is the specific heat of the metal?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
Exactly 313.5 j will raise the temperature of 10.0 g of a metal from 25.0 c to 60.0 c. what is the s...
Questions

Mathematics, 22.05.2020 21:01


Mathematics, 22.05.2020 21:01

Computers and Technology, 22.05.2020 21:01

Mathematics, 22.05.2020 21:01





Mathematics, 22.05.2020 21:01





Mathematics, 22.05.2020 21:01



History, 22.05.2020 21:02


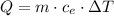 we can clear specific heat:
we can clear specific heat: