HELP PLEASE
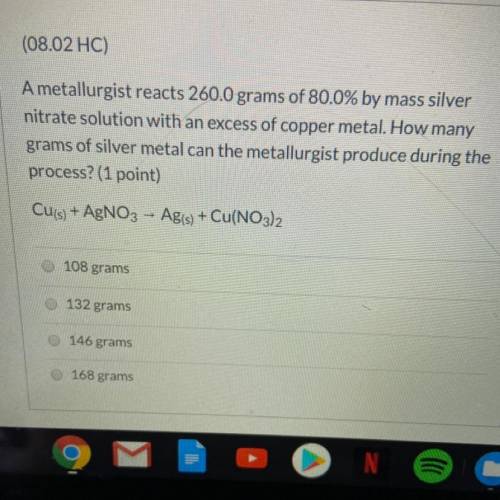
A metallurgist reacts 260.0 grams of 80.0% by mass silver nitrate solution w...

Chemistry, 31.03.2020 18:56 sparkyjones02
HELP PLEASE
A metallurgist reacts 260.0 grams of 80.0% by mass silver nitrate solution with an excess of copper metal. How many grams of silver metal can the metallurgist produce during the process?
Cu(s) + AgNO3 - Ag(s) + Cu(NO3)2
Answers
A.108 grams
B.132 grams
C.146 grams
D.168 grams

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:10
The concept of empiricism states that all rationally accepted knowledge is determined from experience. francis bacon was one of the first scientists to promote this theory. what was it’s impact on society?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1


Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
Questions

Arts, 05.10.2021 04:20

History, 05.10.2021 04:20

Biology, 05.10.2021 04:20



Mathematics, 05.10.2021 04:20





French, 05.10.2021 04:20

History, 05.10.2021 04:20


English, 05.10.2021 04:20








