HELP ME ASAP!
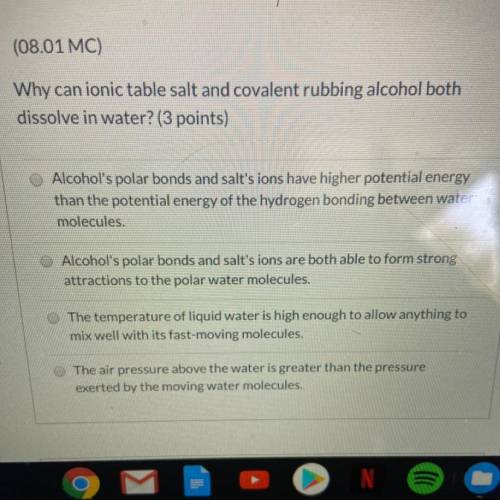
Why can ionic table salt and covalent rubbing alcohol both
dissolve in wat...

HELP ME ASAP!
Why can ionic table salt and covalent rubbing alcohol both
dissolve in water? (3 points)
A. Alcohol's polar bonds and salt's ions have higher potential energy
than the potential energy of the hydrogen bonding between water
molecules.
B. Alcohol's polar bonds and salt's ions are both able to form strong
attractions to the polar water molecules.
C. The temperature of liquid water is high enough to allow anything to
mix well with its fast-moving molecules.
D. The air pressure above the water is greater than the pressure
exerted by the moving water molecules.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1

You know the right answer?
Questions


Mathematics, 02.10.2019 03:00




English, 02.10.2019 03:00
















