
Chemistry, 01.04.2020 01:04 ericadawn2852
William measures a test tube and finds that the mass of the test tube is 5g. He places the lone reactant in the test tube and finds the mass to be 12.5g. He carries out a reaction by heating the test tube. At the end of the reaction, he measures the mass of the test tube containing the lone product and finds it to have a mass of 9.3g. What is the percent yield for this experiment if he expected to produce 5g of product?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1


Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
William measures a test tube and finds that the mass of the test tube is 5g. He places the lone reac...
Questions



English, 13.01.2021 14:00

Mathematics, 13.01.2021 14:00



Physics, 13.01.2021 14:00

Geography, 13.01.2021 14:00












SAT, 13.01.2021 14:00

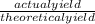 x 100
x 100 x 100
x 100

