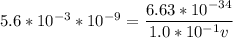Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 12:30
17) large amounts of very important metal titanium are made by reacting magnesium metal with titanium tetrachloride. titanium metal and magnesium chloride are produced. a) write the balanced equation for this reaction. b) how many kilograms of magnesium are required to produce 1.00 kilograms of titanium? ( show work, .)
Answers: 1
You know the right answer?
Calculate the wavelength, in meters, associated with a 1.0 x 10^2 g golf ball moving at 27 m/s (abou...
Questions


History, 23.09.2019 09:50

History, 23.09.2019 09:50

Biology, 23.09.2019 09:50

Mathematics, 23.09.2019 09:50




History, 23.09.2019 09:50



Mathematics, 23.09.2019 09:50

Mathematics, 23.09.2019 09:50

Health, 23.09.2019 09:50


History, 23.09.2019 09:50



Biology, 23.09.2019 09:50

English, 23.09.2019 09:50


 .
.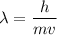
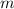 is the mass of the ball,
is the mass of the ball,  is its velocity, and
is its velocity, and 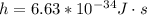 is the plank's constant.
is the plank's constant.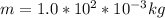
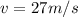 ;
; 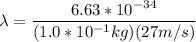

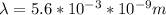 ,
,