
Chemistry, 01.04.2020 05:43 payshencec21
For the equilibrium
2IBr(g) ⇌ I₂(g) + Br₂(g) Kc = 8.50 × 10⁻³ at 150°C.
If 0.0600 mol of IBr is placed in a 1.0-L container, what is the partial pressure of I₂(g) in atm after equilibrium is reached?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2


Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1

Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1
You know the right answer?
For the equilibrium
2IBr(g) ⇌ I₂(g) + Br₂(g) Kc = 8.50 × 10⁻³ at 150°C.
If 0...
2IBr(g) ⇌ I₂(g) + Br₂(g) Kc = 8.50 × 10⁻³ at 150°C.
If 0...
Questions



Mathematics, 17.11.2020 01:40

Health, 17.11.2020 01:40

Mathematics, 17.11.2020 01:40

Mathematics, 17.11.2020 01:40

English, 17.11.2020 01:40


English, 17.11.2020 01:40

Mathematics, 17.11.2020 01:40

Advanced Placement (AP), 17.11.2020 01:40

Mathematics, 17.11.2020 01:40

Chemistry, 17.11.2020 01:40

Mathematics, 17.11.2020 01:40



Health, 17.11.2020 01:40

History, 17.11.2020 01:40


![[IBr]i = \frac{0.0600mol}{1.0L} = 0.060M](/tpl/images/0575/0702/18a8d.png)
![Kc = 8.50 \times 10^{-3} = \frac{[I_2][Br_2]}{[IBr]^{2} } = \frac{x^{2} }{(0.060-x)^{2} } \\\\x = 0.00507](/tpl/images/0575/0702/c2c7d.png)
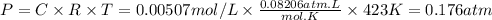
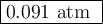
![\text{[IBr]} = \dfrac{\text{0.0600 mol}}{\text{1.0 L}} = \text{0.0600 mol/L}](/tpl/images/0575/0702/bae1e.png)
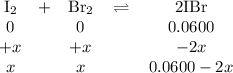
![\begin{array}{rcl}K_{\text{c}}&=&\dfrac{\text{[IBr]}^{2}} {\text{[I$_{2}$][Br]$_{2}$}}\\\\8.50 \times 10^{-2}&=&{\dfrac{(0.0600 - 2x)^{2}}{x^{2}}}& &\\\\0.2915x & = &{\dfrac{0.0600 - 2x}{x}}& &\\\\0.2915x & = &0.0600 - 2x\\\\2.2915x & = & 0.0600\\x & = & \textbf{0.026 18 mol/L}\\\end{array}\\](/tpl/images/0575/0702/36fc0.png)



