Calculate the volume occupied by 272g
of methane at a pressure of 250 kPa and a
temperatur...

Chemistry, 01.04.2020 13:36 genyjoannerubiera
Calculate the volume occupied by 272g
of methane at a pressure of 250 kPa and a
temperature of 54°C.
(R = 8.31JK-1 mol-1; M, methane = 16.0)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3


Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
Questions

Mathematics, 05.11.2019 22:31



History, 05.11.2019 22:31


Mathematics, 05.11.2019 22:31


Mathematics, 05.11.2019 22:31


Spanish, 05.11.2019 22:31



Mathematics, 05.11.2019 22:31






Mathematics, 05.11.2019 22:31

English, 05.11.2019 22:31

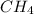 ) = 272 grams
) = 272 grams 

