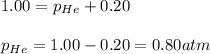The gas that a diver breathes must be maintained at a partial pressure of 0.20 atm of oxygen. More oxygen could poison the diver, and less would lead to suffocation. The total pressure, however, must be equal to the external pressure to avoid collapsing the lungs. A special valve is used to equalize the pressure inside the diver's lungs with the external pressure by adding helium gas. The valve also maintains oxygen levels by using Dalton's Law. If the total pressure in the cylinder is 1.00 atm, what is the pressure of the helium?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
The gas that a diver breathes must be maintained at a partial pressure of 0.20 atm of oxygen. More o...
Questions

Spanish, 30.01.2021 08:10


English, 30.01.2021 08:10

Physics, 30.01.2021 08:10

Mathematics, 30.01.2021 08:10

Mathematics, 30.01.2021 08:10

Biology, 30.01.2021 08:10

History, 30.01.2021 08:10

History, 30.01.2021 08:10


Physics, 30.01.2021 08:10

Mathematics, 30.01.2021 08:10

English, 30.01.2021 08:10

History, 30.01.2021 08:10

Mathematics, 30.01.2021 08:10


Mathematics, 30.01.2021 08:10

Mathematics, 30.01.2021 08:10

History, 30.01.2021 08:10

Physics, 30.01.2021 08:10

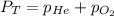
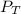 = 1.00 atm
= 1.00 atm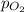 = 0.20 atm
= 0.20 atm