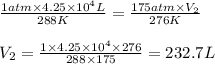Chemistry, 01.04.2020 16:58 jjjjjjgegi3088
An airtight container with a volume of 4.25 x 10^4L, an internal pressure of 1.00 atm, and an internal temperature of 15.00 *C is washed off the deck of a ship and sinks to a depth where the pressure is 175 atm and the temperature is 3.00 *C. What will the volume of the gas inside be when the container breaks under the pressure at this depth?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
An airtight container with a volume of 4.25 x 10^4L, an internal pressure of 1.00 atm, and an intern...
Questions

Chemistry, 30.11.2019 06:31




Chemistry, 30.11.2019 06:31

Mathematics, 30.11.2019 06:31

Social Studies, 30.11.2019 06:31

Physics, 30.11.2019 06:31

Mathematics, 30.11.2019 06:31


Chemistry, 30.11.2019 06:31



English, 30.11.2019 06:31



Mathematics, 30.11.2019 06:31


Arts, 30.11.2019 06:31

Mathematics, 30.11.2019 06:31

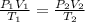
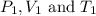 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas 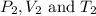 are the final pressure, volume and temperature of the gas
are the final pressure, volume and temperature of the gas![P_1=1.00atm\\V_1=4.25\times 10^4L\\T_1=15^oC=[15+273]K=288K\\P_2=175atm\\V_2=?\\T_2=3^oC=[3+273]K=276K](/tpl/images/0575/4914/4be35.png)