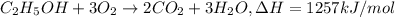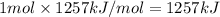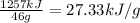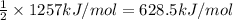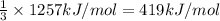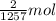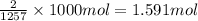Chemistry, 01.04.2020 17:26 KariSupreme
Reaction enthalpy for ethanol oxidation, C2H5OH+3O2 -> 2CO2 + 3H2O is 1257 kJ/mole. Energy content per mol fuel kJ Energy content per gram fuel = kJ Energy released per mol CO2 formed kJ Energy released per mol O2 consumed= kJ Moles of CO2 formed per 1000 kJ energy released

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Reaction enthalpy for ethanol oxidation, C2H5OH+3O2 -> 2CO2 + 3H2O is 1257 kJ/mole. Energy conten...
Questions

Mathematics, 20.11.2020 06:00

Computers and Technology, 20.11.2020 06:00

Mathematics, 20.11.2020 06:00



English, 20.11.2020 06:00

Chemistry, 20.11.2020 06:00

Mathematics, 20.11.2020 06:00

Mathematics, 20.11.2020 06:00


Mathematics, 20.11.2020 06:00

Mathematics, 20.11.2020 06:00

Mathematics, 20.11.2020 06:00

Mathematics, 20.11.2020 06:00

Mathematics, 20.11.2020 06:00

Biology, 20.11.2020 06:00

Arts, 20.11.2020 06:00

History, 20.11.2020 06:00

Mathematics, 20.11.2020 06:00