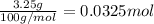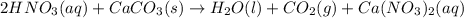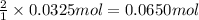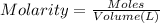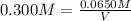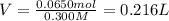Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1


Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
You know the right answer?
How many milliliters of 0.300 M nitric acid are required to react completely with 3.25 g of calcium...
Questions

History, 06.11.2020 04:20

Mathematics, 06.11.2020 04:20

Chemistry, 06.11.2020 04:20

Social Studies, 06.11.2020 04:20


Mathematics, 06.11.2020 04:20



History, 06.11.2020 04:20


Mathematics, 06.11.2020 04:20


Social Studies, 06.11.2020 04:20




History, 06.11.2020 04:20


Business, 06.11.2020 04:20