
Chemistry, 01.04.2020 19:20 taylor5384
What quantity, in moles, of oxygen is consumed when 717.4 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g)? H2(g) + O2(g) → H2O(l); ΔH° = –285.8 kJ

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
What quantity, in moles, of oxygen is consumed when 717.4 kJ of energy is evolved from the combustio...
Questions

Mathematics, 21.12.2019 16:31

Social Studies, 21.12.2019 16:31

History, 21.12.2019 16:31

English, 21.12.2019 16:31


History, 21.12.2019 16:31



Mathematics, 21.12.2019 16:31

Biology, 21.12.2019 16:31


Biology, 21.12.2019 16:31

English, 21.12.2019 16:31

Mathematics, 21.12.2019 16:31

English, 21.12.2019 16:31





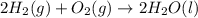
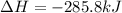
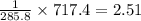 moles
moles

