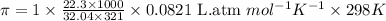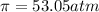Chemistry, 01.04.2020 19:31 kjjackson012002
What is the osmotic pressure of a solution made from 22.3 g of methanol (MM = 32.04 g/mol) that was added to water to make 321 mL of solution at 25.0 °C?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 13:30
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. a + b yields products trial [a] [b] rate 1 0.30 m 0.25 m 1.2 × 10-2 m/min 2 0.30 m 0.50 m 4.8 × 10-2 m/min 3 0.60 m 0.50 m 9.6 × 10-2 m/min
Answers: 1
You know the right answer?
What is the osmotic pressure of a solution made from 22.3 g of methanol (MM = 32.04 g/mol) that was...
Questions






Mathematics, 30.07.2019 19:30

Business, 30.07.2019 19:30

Mathematics, 30.07.2019 19:30


Health, 30.07.2019 19:30

History, 30.07.2019 19:30


Biology, 30.07.2019 19:30




Biology, 30.07.2019 19:30



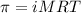
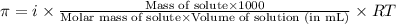
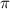 = osmotic pressure of the solution = ?
= osmotic pressure of the solution = ?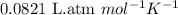
![25^oC=[273+25]=298K](/tpl/images/0575/9078/6a9f9.png)