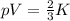Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
Using the kinetic molecular theory, explain what the difference is between a gas that exerts a press...
Questions

Business, 14.01.2022 08:50



Mathematics, 14.01.2022 08:50



Health, 14.01.2022 08:50



Mathematics, 14.01.2022 08:50

Computers and Technology, 14.01.2022 08:50

English, 14.01.2022 08:50

Mathematics, 14.01.2022 09:00

Mathematics, 14.01.2022 09:00

Business, 14.01.2022 09:00


History, 14.01.2022 09:00

Mathematics, 14.01.2022 09:00

Mathematics, 14.01.2022 09:00