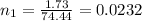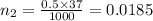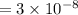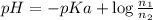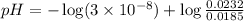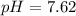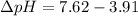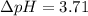Chemistry, 01.04.2020 22:32 shenzhen10
The pH of 0.50 M HClO is 3.91. Calculate the change in pH when 1.73 g of NaClO (FW = 74.44 g/mol) is added to 40 mL of 0.50 M HClO (FW = 52.46 g/mol). Ignore any changes in volume. The Ka value for HClO is 3.0 x 10-8.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Why should the scientific method be used to answer a question? a. it provides a way to test an idea without any bias. b. it provides a way to test a hypothesis. c. it provides a way to ensure all hypotheses are proven correct. d. it provides a way to quickly turn a hypothesis into a scientific theory.
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 23.06.2019 17:00
What statement about the energy of a phase change is true
Answers: 1
You know the right answer?
The pH of 0.50 M HClO is 3.91. Calculate the change in pH when 1.73 g of NaClO (FW = 74.44 g/mol) is...
Questions


Mathematics, 06.06.2020 05:00




Mathematics, 06.06.2020 05:00


Biology, 06.06.2020 05:00


Mathematics, 06.06.2020 05:00



Mathematics, 06.06.2020 05:00



Mathematics, 06.06.2020 05:00



History, 06.06.2020 05:00