
Chemistry, 01.04.2020 22:29 ashleyc2442
Given the reaction at equilibrium n2(g) + 3h2(g) ↔ 2nh3(g) increasing the concentration of N2(g) will increase the forward reaction rate due to 1. A decrease in the number of effective collisions

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

You know the right answer?
Given the reaction at equilibrium n2(g) + 3h2(g) ↔ 2nh3(g) increasing the concentration of N2(g) wil...
Questions

Mathematics, 13.10.2020 21:01




Chemistry, 13.10.2020 21:01

History, 13.10.2020 21:01


Mathematics, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01

English, 13.10.2020 21:01

Geography, 13.10.2020 21:01


Health, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01





Mathematics, 13.10.2020 21:01

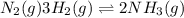 an increase in
an increase in 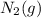 means an increase in the number of reactants.
means an increase in the number of reactants. 

