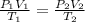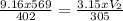A gas at 928 kpa, 129 C occupies a volume of 569 L. Calculate the volume at 319 kpa and
32 C....

Chemistry, 02.04.2020 01:32 aprilayleen
A gas at 928 kpa, 129 C occupies a volume of 569 L. Calculate the volume at 319 kpa and
32 C.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Questions



Mathematics, 16.10.2020 09:01



History, 16.10.2020 09:01






Mathematics, 16.10.2020 09:01




Mathematics, 16.10.2020 09:01

History, 16.10.2020 09:01


English, 16.10.2020 09:01

Mathematics, 16.10.2020 09:01