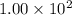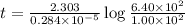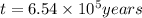Radioactive plutonium−239 (t1/2 = 2.44 × 105 yr) is used in nuclear reactors and atomic bombs. If there are 6.40 × 102 g of the isotope in a small atomic bomb, how long will it take for the substance to decay to 1.00 × 102 g, too small an amount for an effective bomb? (Hint: Radioactive decays follow first-order kinetics.)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Radioactive plutonium−239 (t1/2 = 2.44 × 105 yr) is used in nuclear reactors and atomic bombs. If th...
Questions

History, 23.04.2021 21:30

Mathematics, 23.04.2021 21:30

Mathematics, 23.04.2021 21:30


Mathematics, 23.04.2021 21:30

Mathematics, 23.04.2021 21:30


Chemistry, 23.04.2021 21:30



Mathematics, 23.04.2021 21:30

Mathematics, 23.04.2021 21:30



Mathematics, 23.04.2021 21:30

Mathematics, 23.04.2021 21:30

Mathematics, 23.04.2021 21:30

English, 23.04.2021 21:30


History, 23.04.2021 21:30

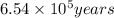
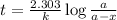
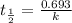
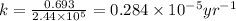
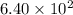 g of the isotope to decay to
g of the isotope to decay to