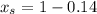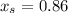Chemistry, 02.04.2020 03:35 hdjsjfjruejchhehd
6.55. A vapor stream that is 65 mole% styrene and 35 mole% toluene is in equilibrium with a liquid mixture of the same two species. The pressure in the system is 150 mm Hg absolute. Use Raoult’s law to estimate the composition of the liquid and the system temperature

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the h+ concentration for an aqueous solution with poh = 4.01 at 25 ∘c? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1


Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
6.55. A vapor stream that is 65 mole% styrene and 35 mole% toluene is in equilibrium with a liquid m...
Questions






Mathematics, 09.02.2021 17:20

Arts, 09.02.2021 17:20

Mathematics, 09.02.2021 17:20

History, 09.02.2021 17:20

History, 09.02.2021 17:20



Mathematics, 09.02.2021 17:20

Mathematics, 09.02.2021 17:20

Mathematics, 09.02.2021 17:20

English, 09.02.2021 17:20




Mathematics, 09.02.2021 17:20

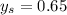
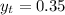
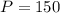 mm Hg
mm Hg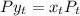
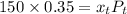
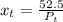
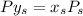
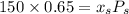
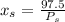
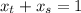
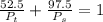
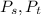 are dependent on temperature.
are dependent on temperature.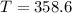 K, value of
K, value of 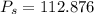 mm Hg and
mm Hg and 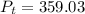 mm Hg
mm Hg