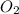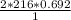2HgO(s) -> 2Hg(l) + O2(g)

Chemistry, 02.04.2020 09:43 jamesnech66
What mass of HgO is required to produce 0.692 mol of O2?
2HgO(s) -> 2Hg(l) + O2(g)


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

You know the right answer?
What mass of HgO is required to produce 0.692 mol of O2?
2HgO(s) -> 2Hg(l) + O2(g)
2HgO(s) -> 2Hg(l) + O2(g)
Questions

Mathematics, 26.07.2021 23:50

Mathematics, 26.07.2021 23:50


English, 26.07.2021 23:50

Spanish, 26.07.2021 23:50

Law, 26.07.2021 23:50


Social Studies, 27.07.2021 01:00

Law, 27.07.2021 01:00


English, 27.07.2021 01:00

Engineering, 27.07.2021 01:00


Mathematics, 27.07.2021 01:00



Biology, 27.07.2021 01:00

Mathematics, 27.07.2021 01:00


Mathematics, 27.07.2021 01:00