
Chemistry, 02.04.2020 22:14 bobbyhill24
A 7.11g sample of potassium chlorate was decomposed according to the following equation: 2KCLO3 -->2KCL + 3O2 How many moles of oxygen are formed?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2


Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
You know the right answer?
A 7.11g sample of potassium chlorate was decomposed according to the following equation: 2KCLO3 --&g...
Questions


English, 19.11.2019 05:31

Mathematics, 19.11.2019 05:31


Mathematics, 19.11.2019 05:31

Biology, 19.11.2019 05:31

Mathematics, 19.11.2019 05:31

Mathematics, 19.11.2019 05:31


Mathematics, 19.11.2019 05:31




Mathematics, 19.11.2019 05:31

Computers and Technology, 19.11.2019 05:31



History, 19.11.2019 05:31


History, 19.11.2019 05:31

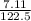
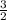 × 0.0580 moles
× 0.0580 moles

