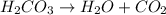Chemistry, 30.10.2019 08:31 jngonzo1226
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, it forms magnesium nitrate, mg(no3)2, and carbonic acid, h2co3. carbonic acid then breaks down into water and carbon dioxide. which two types of reactions take place in this process?
a.) synthesis and decomposition
b.) single-replacement and combustion
c.) double-replacement and decomposition
d.) double-replacement and combustion

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1


Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
You know the right answer?
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, it forms magnesium nitrate, mg(no3)2...
Questions

Physics, 10.10.2019 15:00

History, 10.10.2019 15:00

Social Studies, 10.10.2019 15:00

History, 10.10.2019 15:00


History, 10.10.2019 15:00


Mathematics, 10.10.2019 15:00

Biology, 10.10.2019 15:00

History, 10.10.2019 15:00

Physics, 10.10.2019 15:00

Spanish, 10.10.2019 15:00

English, 10.10.2019 15:00

Computers and Technology, 10.10.2019 15:00

Mathematics, 10.10.2019 15:00



Biology, 10.10.2019 15:00


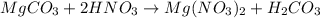 : is a double displacement reaction in which ion exchange takes place.
: is a double displacement reaction in which ion exchange takes place.