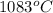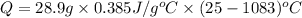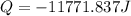Chemistry, 29.12.2019 18:31 jeffhuffle17
Calculate the energy released when a 28.9 gram piece of paper is cooled from its melting point of 1083 degrees celsius to 25.0 degrees celsius. the specific heat of copper is .385 j/g celsius.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
Calculate the energy released when a 28.9 gram piece of paper is cooled from its melting point of 10...
Questions

Mathematics, 14.04.2021 21:50

Biology, 14.04.2021 21:50

Social Studies, 14.04.2021 21:50

Health, 14.04.2021 21:50


Mathematics, 14.04.2021 21:50


Computers and Technology, 14.04.2021 21:50

Mathematics, 14.04.2021 21:50

Mathematics, 14.04.2021 21:50

English, 14.04.2021 21:50

Mathematics, 14.04.2021 21:50


Mathematics, 14.04.2021 21:50

Mathematics, 14.04.2021 21:50





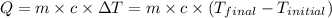
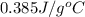
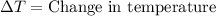
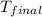 = final temperature =
= final temperature = 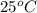
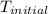 = initial temperature =
= initial temperature =