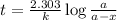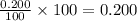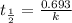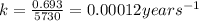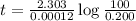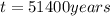Chemistry, 03.04.2020 05:02 jayrichesz80Jahree
The half-life of radioactive carbon-14 is 5730 years. If the 14C level in a sample of organic matter has been reduced to 0.200% of its original value, approximately how much time has passed? Radioactive decay follows first-order kinetics.
a. 1650 years
b. 51,400 years
c. 29,900 years
d 2,870,000 years
e. 9220 years

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

You know the right answer?
The half-life of radioactive carbon-14 is 5730 years. If the 14C level in a sample of organic matter...
Questions

Biology, 02.03.2021 20:20


Mathematics, 02.03.2021 20:20

Mathematics, 02.03.2021 20:20

English, 02.03.2021 20:20


Social Studies, 02.03.2021 20:20


Biology, 02.03.2021 20:20


Spanish, 02.03.2021 20:20


English, 02.03.2021 20:20

Mathematics, 02.03.2021 20:20



Social Studies, 02.03.2021 20:20


Mathematics, 02.03.2021 20:20

Social Studies, 02.03.2021 20:20