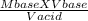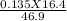Chemistry, 03.04.2020 05:31 dshood2298
16.4 mL of 0.135 M NaOH is used to neutralize 46.9 mL of HCl. The concentration of the HCl solution is M (3 decimal places).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1

Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1
You know the right answer?
16.4 mL of 0.135 M NaOH is used to neutralize 46.9 mL of HCl. The concentration of the HCl solution...
Questions

Mathematics, 30.11.2020 05:20



Mathematics, 30.11.2020 05:20


History, 30.11.2020 05:20

Social Studies, 30.11.2020 05:20




Mathematics, 30.11.2020 05:20

Chemistry, 30.11.2020 05:20






Mathematics, 30.11.2020 05:20

Chemistry, 30.11.2020 05:20

History, 30.11.2020 05:20