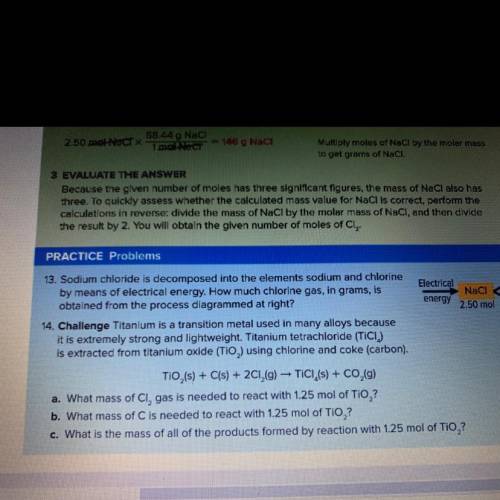
What is the mass of all the products formed by the reaction with 1.25 mol of TiO2?
PLEASE HELP...

Chemistry, 03.04.2020 06:05 chayaharroch03
What is the mass of all the products formed by the reaction with 1.25 mol of TiO2?
PLEASE HELP ASAP

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
You know the right answer?
Questions

Mathematics, 23.09.2020 09:01

Mathematics, 23.09.2020 09:01

Mathematics, 23.09.2020 09:01

Social Studies, 23.09.2020 09:01

Mathematics, 23.09.2020 09:01

Mathematics, 23.09.2020 09:01

Mathematics, 23.09.2020 09:01


Social Studies, 23.09.2020 09:01

History, 23.09.2020 09:01


Mathematics, 23.09.2020 09:01

Mathematics, 23.09.2020 09:01

English, 23.09.2020 09:01

Mathematics, 23.09.2020 09:01

History, 23.09.2020 09:01

English, 23.09.2020 09:01


Social Studies, 23.09.2020 09:01



