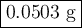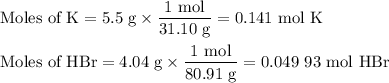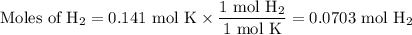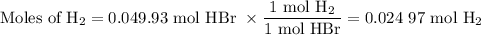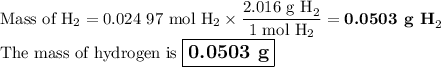Chemistry, 03.04.2020 06:24 spdesch2558
2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hydrogen gas(H₂)
●a). What is the limiting reactant?
●b.)What is the excess reactant?
●C.)How much product is produced?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1


Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hyd...
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hyd...
Questions

Arts, 05.11.2020 22:50




Mathematics, 05.11.2020 22:50

History, 05.11.2020 22:50


Mathematics, 05.11.2020 22:50

History, 05.11.2020 22:50







Mathematics, 05.11.2020 22:50

Mathematics, 05.11.2020 22:50



Mathematics, 05.11.2020 22:50