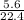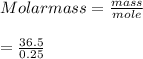Chemistry, 03.04.2020 20:41 22chandlerlashley
A 0.500 g sample of He at STP has a volume that is one-half that of an unknown pure gas also at STP. The unknown pure gas sample has a mass of 36.5 g. What is the molar mass of the unknown gas?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
You know the right answer?
A 0.500 g sample of He at STP has a volume that is one-half that of an unknown pure gas also at STP....
Questions

Mathematics, 03.09.2021 19:40

Mathematics, 03.09.2021 19:40


Spanish, 03.09.2021 19:40

Mathematics, 03.09.2021 19:40


Mathematics, 03.09.2021 19:40


Mathematics, 03.09.2021 19:40

Biology, 03.09.2021 19:40

Mathematics, 03.09.2021 19:40

Mathematics, 03.09.2021 19:40

Spanish, 03.09.2021 19:40

Mathematics, 03.09.2021 19:40

Mathematics, 03.09.2021 19:40

Mathematics, 03.09.2021 19:40

Mathematics, 03.09.2021 19:40

Mathematics, 03.09.2021 19:40


Biology, 03.09.2021 19:40

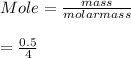 Mole of He = 0.125 moleNext, we shall determine the volume occupied by 0.125 mole of He at stp
Mole of He = 0.125 moleNext, we shall determine the volume occupied by 0.125 mole of He at stp