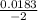Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3


Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
You know the right answer?
H2SO4 is a strong acid because the first proton ionizes 100%. The Ka of the second proton is 1.1x10-...
Questions



English, 22.12.2020 22:30



Mathematics, 22.12.2020 22:30




Mathematics, 22.12.2020 22:30

Arts, 22.12.2020 22:30


Mathematics, 22.12.2020 22:30

Mathematics, 22.12.2020 22:30

Mathematics, 22.12.2020 22:30


Mathematics, 22.12.2020 22:30

Business, 22.12.2020 22:30

Chemistry, 22.12.2020 22:30

Mathematics, 22.12.2020 22:30

![\frac{[SO42-] [H3O+]}{[HSO4-]}](/tpl/images/0580/8225/cd1fe.png)
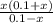
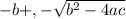 / 2a
/ 2a![\sqrt[-(-o.111)]{(-0.111)^2 - 4(-1) (0.0011) }](/tpl/images/0580/8225/19ba8.png) / 2(-1)
/ 2(-1)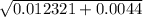 / -2
/ -2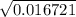 / -2
/ -2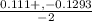
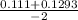 , x =
, x = 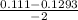
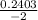 , x =
, x =