
Chemistry, 04.04.2020 15:01 janeou17xn
Be sure to answer all parts. A sample of natural gas contains 6.816 moles of methane (CH4), 0.589 moles of ethane (C2H6), and 0.381 moles of propane (C3H8). If the total pressure of the gases is 3.93 atm, what are the partial pressures of the gases?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Be sure to answer all parts. A sample of natural gas contains 6.816 moles of methane (CH4), 0.589 mo...
Questions




History, 31.01.2020 01:05

Mathematics, 31.01.2020 01:05

Mathematics, 31.01.2020 01:05




Social Studies, 31.01.2020 01:05



History, 31.01.2020 01:05

Biology, 31.01.2020 01:05

Mathematics, 31.01.2020 01:05

Spanish, 31.01.2020 01:05

History, 31.01.2020 01:05


Mathematics, 31.01.2020 01:05

Chemistry, 31.01.2020 01:05

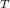 = P
= P + P
+ P + P
+ P ...............
...............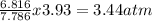
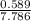 x 3.93 = 0.30atm
x 3.93 = 0.30atm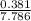 x 3.93 = 0.19atm
x 3.93 = 0.19atm

