
Chemistry, 04.04.2020 09:19 keyshawn437
To make use of an ionic hydrate for storing solar energy, you place 422.0 kg of sodium sulfate decahydrate on your house roof. Assuming complete reaction and 100% efficiency of heat transfer, how much heat (in kJ) is released to your house at night

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1


Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 23.06.2019 08:10
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
You know the right answer?
To make use of an ionic hydrate for storing solar energy, you place 422.0 kg of sodium sulfate decah...
Questions

Spanish, 07.09.2020 05:01

Mathematics, 07.09.2020 05:01

Biology, 07.09.2020 05:01

Mathematics, 07.09.2020 05:01

Mathematics, 07.09.2020 05:01

English, 07.09.2020 05:01

History, 07.09.2020 05:01

Mathematics, 07.09.2020 05:01

Mathematics, 07.09.2020 05:01

English, 07.09.2020 05:01

Biology, 07.09.2020 05:01

Mathematics, 07.09.2020 05:01

Mathematics, 07.09.2020 05:01





Chemistry, 07.09.2020 05:01

Mathematics, 07.09.2020 05:01

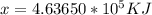
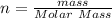
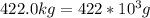 for mass of sodium sulfate decahydrate(
for mass of sodium sulfate decahydrate(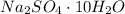 ),
), 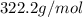 (This value is a constant )for the molar mass of sodium sulfate decahydrate
(This value is a constant )for the molar mass of sodium sulfate decahydrate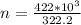
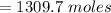
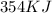 of energy
of energy 

