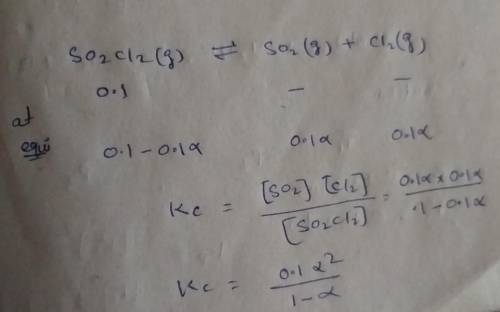Chemistry, 04.04.2020 09:40 kolbehoneyman
Consider the reaction below. Initially the concentration of SO2Cl2 is 0.1000 M. Solve for the equilibrium concentration of SO2Cl2((g). SO2Cl2(g) ←⎯⎯→ SO2(g) + Cl2(g) Kc = 2.99 x 10-7 at 227 °C

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
Consider the reaction below. Initially the concentration of SO2Cl2 is 0.1000 M. Solve for the equili...
Questions

Mathematics, 09.04.2021 22:50

Mathematics, 09.04.2021 22:50


Mathematics, 09.04.2021 22:50


Mathematics, 09.04.2021 22:50


Advanced Placement (AP), 09.04.2021 22:50

Mathematics, 09.04.2021 22:50


History, 09.04.2021 22:50



Mathematics, 09.04.2021 22:50

Social Studies, 09.04.2021 22:50

Mathematics, 09.04.2021 22:50


Mathematics, 09.04.2021 22:50

Physics, 09.04.2021 22:50

Mathematics, 09.04.2021 22:50

![[SO_2Cl_2] = 0.09983 M](/tpl/images/0582/0550/0c89a.png)
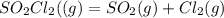
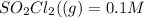
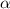
![[SO_2Cl_2] = 0.1-0.1\alpha](/tpl/images/0582/0550/ed240.png)
![[SO_2] = 0.1\alpha](/tpl/images/0582/0550/97c2e.png)
![[Cl_2] = 0.1\alpha](/tpl/images/0582/0550/13ee9.png)
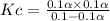
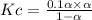

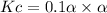
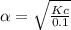
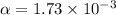
![[SO_2Cl_2] = 0.1-0.1\alpha = 0.1-0.1\times 0.00173](/tpl/images/0582/0550/6431a.png)