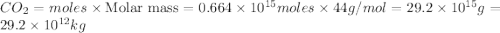Assume that all of this fossil fuel is in the form of octane (C8H18) and calculate how much CO2 in kilograms is produced by world fossil fuel combustion per year. (Hint: Begin by writing a balanced equation for the combustion of octane.) Express your answer using two significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
Assume that all of this fossil fuel is in the form of octane (C8H18) and calculate how much CO2 in k...
Questions











History, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01



Biology, 12.10.2020 21:01


Social Studies, 12.10.2020 21:01

Social Studies, 12.10.2020 21:01


Advanced Placement (AP), 12.10.2020 21:01

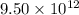 kg of petroleum per year. Assume that all of this petroleum is in the form of octane. Calculate how much CO2 in kilograms is produced by world fossil fuel combustion per year.( Hint: Begin by writing a balanced equation for the combustion of octane.)
kg of petroleum per year. Assume that all of this petroleum is in the form of octane. Calculate how much CO2 in kilograms is produced by world fossil fuel combustion per year.( Hint: Begin by writing a balanced equation for the combustion of octane.)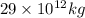
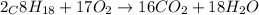
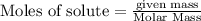
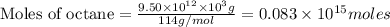
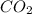
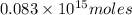 of octane give =
of octane give =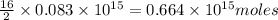 of
of