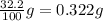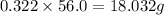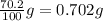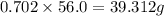Chemistry, 04.04.2020 10:59 iklassibrahim123
Consider the solubilities of a particular solute at two different temperatures. Temperature ( ∘ C ) Solubility ( g / 100 g H 2 O ) 20.0 32.2 30.0 70.2 Suppose a saturated solution of this solute was made using 56.0 g H 2 O at 20.0 °C. How much more solute can be added if the temperature is increased to 30.0 ∘ C? mass:

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

You know the right answer?
Consider the solubilities of a particular solute at two different temperatures. Temperature ( ∘ C )...
Questions















Computers and Technology, 08.01.2020 02:31

Advanced Placement (AP), 08.01.2020 02:31