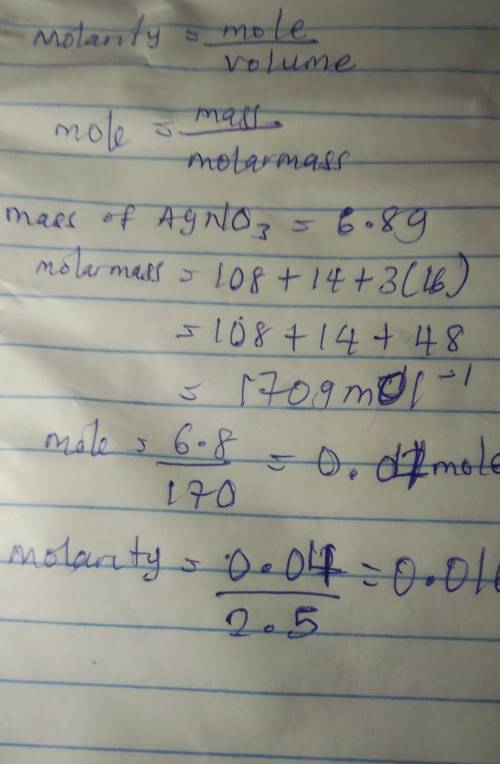Chemistry, 04.04.2020 11:03 capricorn0115
Calculate the molarity of a solution prepared by dissolving 6.80 grams of AgNO3 in 2.50 liters of solution.
SHOW WORK:)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
Calculate the molarity of a solution prepared by dissolving 6.80 grams of AgNO3 in 2.50 liters of so...
Questions

Mathematics, 04.06.2021 07:40

History, 04.06.2021 07:40


Mathematics, 04.06.2021 07:40

English, 04.06.2021 07:40


Mathematics, 04.06.2021 07:50

Mathematics, 04.06.2021 07:50


Mathematics, 04.06.2021 07:50


Arts, 04.06.2021 07:50

Mathematics, 04.06.2021 07:50




Mathematics, 04.06.2021 07:50



Mathematics, 04.06.2021 07:50