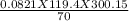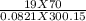A 70-liter tank of oxygen gas at 27 degree Celcius and 42.0 atm springs a leak overnight. when the tank was found in the morning, the pressure in the tank had dropped to 19.0 atm. If the tank originally held 119.4 moles of oxygen gas, how many moles of gas were left the next morning, assuming the temperature and volume of the rank stayed constant?.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
A 70-liter tank of oxygen gas at 27 degree Celcius and 42.0 atm springs a leak overnight. when the t...
Questions





English, 07.04.2020 22:45

Chemistry, 07.04.2020 22:45




Mathematics, 07.04.2020 22:46

Health, 07.04.2020 22:46



History, 07.04.2020 22:46


English, 07.04.2020 22:46

Computers and Technology, 07.04.2020 22:46


Mathematics, 07.04.2020 22:46

Mathematics, 07.04.2020 22:46