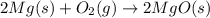Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3.97 g of magnesium ribbon burns with 8.05 g of oxygen, a bright, white light and a white, powdery product are formed. Enter the balanced chemical equation for this reaction. Be sure to include all physical states.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2


Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3...
Questions



Computers and Technology, 24.02.2020 21:32


Chemistry, 24.02.2020 21:32


English, 24.02.2020 21:32

Health, 24.02.2020 21:32




Mathematics, 24.02.2020 21:33


Chemistry, 24.02.2020 21:33

Chemistry, 24.02.2020 21:33



Mathematics, 24.02.2020 21:33

Mathematics, 24.02.2020 21:33

Mathematics, 24.02.2020 21:33