
Chemistry, 04.04.2020 11:51 qdogisbeast6132
What is the pH of a buffer that consists of 0.254 M CH3CH2COONa and 0.329 M CH3CH2COOH? Ka of propanoic acid, CH3CH2COOH is 1.3 x 10-5 Enter your answer with two decimal places.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
You know the right answer?
What is the pH of a buffer that consists of 0.254 M CH3CH2COONa and 0.329 M CH3CH2COOH? Ka of propan...
Questions





History, 16.09.2019 11:30


History, 16.09.2019 11:30


Chemistry, 16.09.2019 11:30

Business, 16.09.2019 11:30


Chemistry, 16.09.2019 11:30

Social Studies, 16.09.2019 11:30




Mathematics, 16.09.2019 11:30

English, 16.09.2019 11:30

Social Studies, 16.09.2019 11:30

Mathematics, 16.09.2019 11:30

![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0582/3078/e4eea.png)
![pH=-\log[K_a]+\log(\frac{[salt]}{[acid]})](/tpl/images/0582/3078/241de.png)
![pH=-\log[K_a]+\log(\frac{[CH_3CH_2COONa]}{[CH_3CH_2COOH]})](/tpl/images/0582/3078/3bb21.png)
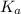 = Dissociation constant of propanoic acid =
= Dissociation constant of propanoic acid = 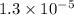
![[CH_3CH_2COONa]=0.254 M](/tpl/images/0582/3078/1c6b0.png)
![[CH_3CH_2COOH]=0.329 M](/tpl/images/0582/3078/2d813.png)
![pH=-\log[1.3\times 10^{-5}]+\log(\frac{[0.254 M]}{[0.329]})](/tpl/images/0582/3078/2a2b4.png)


