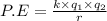Assuming that the distances between the two ions are the same in all cases, which of the following ion pairs has the greatest electrostatic potential energy (i. e., largest in magnitude)? Please explain your answer. a.) Na+ - Cl- b.) Na+ - O-2. c.) Al+3 - O-2. d.) Mg+2-O-2 e.) Na- -Mg+2

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Assuming that the distances between the two ions are the same in all cases, which of the following i...
Questions





Business, 07.03.2020 02:33



Computers and Technology, 07.03.2020 02:33










Mathematics, 07.03.2020 02:33

Health, 07.03.2020 02:33